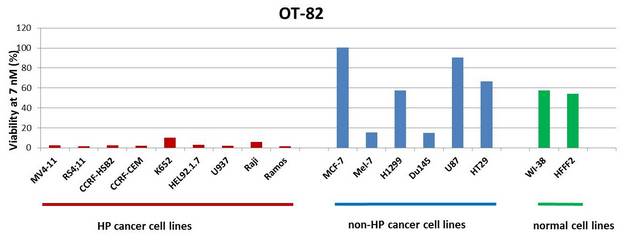
After chemical optimization of original hits, OT-82 preserved selectivity towards hematological tumors.
Efficacy of OT-82 in MV4-11 SC xenograft model (PO administration)
Mice: SCID; Doses: 10, 25, 50mg/kg 6 days a week; Formulation: 30% captisol; 10 mice per group
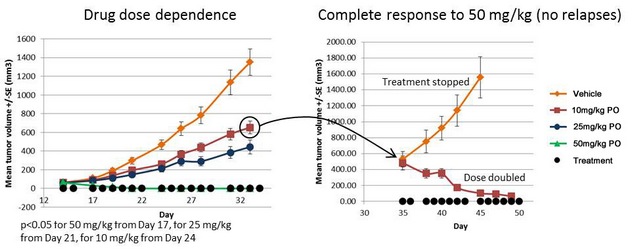
Lack of toxicity in the entire efficacy range

Efficacy of OT-82 in preclinical models (summary)

OT-82 Mechanism of Action
Although compounds that led to OT-82 were found via phenotypical cell based screening of chemical libraries, its mechanism of action was elucidated and confirmed through a combination of shRNA screening and affinity chromatography approach. OT-82 is an NAMPT (NicotinAMide Phosphoribosyl Transferase) inhibitor.
NAMPT catalyzes conversion of nicotinamide to nicotinamide mononucleotide (NMN), a precursor of NAD. That determines its key role in bioenergetics of the cell and in the activity of NAD-dependent proteins, such as sirtuins, PARPS, etc. NAMPT exists in intracellular and extracellular forms. In the cell NAMPT is present in the cytoplasm and nucleus. Its phosphorylated form has 100-times higher activity. The role of other post-translational modifications is not defined yet.
Suggested mechanism of NAMPT participation in leukemia cells proliferation and survival
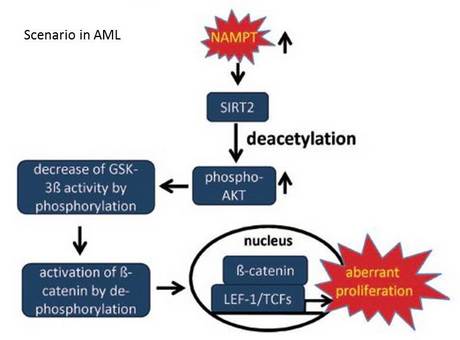
Elevated levels of NAMPT are involved in aberrant proliferation of AML blasts by sequential activation of SIRT2, further elevated by deacetylation, phosphorylation and activation of AKT with subsequent phosphorylation/deactivation of GSK3β. This leads to activation and nuclear accumulation of the proto-oncogene, β-catenin. Nuclear β-catenin binds its co-factors LEF-1/TCF transcription factors and by this activates target genes (e.g. c-myc, cyclin D1 and survivin) involved in cell proliferation as well as in the regulation of cell cycle and apoptosis, which may contribute to aberrant proliferation of AML blasts.
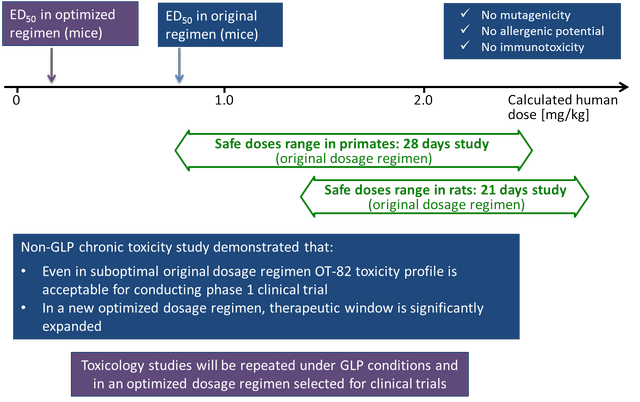
Estimating a safe human dose range of OT-82 in phase I clinical trials based on non-GLP chronic toxicity studies in rats and monkeys
Chronic toxicity studies were conducted using the same dose regimen that was utilized in all in vivo experiments of the program. While these studies were running, a new optimized dose regimen was worked out that has a markedly improved therapeutic index. GLP tox studies and then human clinical trials will be performed using this optimized regimen that significantly lowers the probability of dose related toxicity.
Competitive landscape and OT-82 advantage
NAMPRT has been recognized as an attractive cancer treatment target and several biotech companies attempted to develop NAMPRT inhibitors. Currently, three inhibitors of different chemical classes, APO866 (aka FK866), CHS-828 and GMX1777, have reached Phase I and II clinical trials. Development of all three have been, however, hampered by insufficient therapeutic index. OT-82 developed by OncoTartis has several advantages:
* A von Heideman, et. Cancer Chemother Pharmacol (2010) 65: 1165-1172
* P Hovstadius, et. Clinical Cancer Research (2002) 8: 2843
** K Holen, et., Invest New Drugs (2008) 26: 45-51
*** MJ Pishvaian, et. Journal of Clinical Oncology (2009), ASCO Annual Meeting Proceeding, v.27 15S, 3581
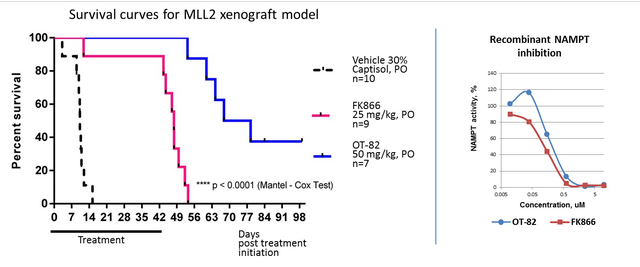
In addition, although NAMPT inhibition potency of OT-82 is similar to other known inhibitors in vitro, it demonstrated higher potency in an in vivo pre-B cells acute lymphoblastic leukemia (ALL) xenografts. This test was run at the Children's Cancer Institute Australia for Medical Research (CCIA) according to the NCI-supported Preclinical Testing Program protocol established for evaluation of new agents against solid tumors and leukemia models.
OT-82 Development timeline
